Background. High risk (HR) cytogenetic remains of poor prognosis, particularly in the RRMM setting. IFM 2010-02 studied pomalidomide and dexamethasone (Pd) and demonstrated limited activity, with a median TTP overall at 5.5 months, and at 7.3 vs 2.8 months in HR RRMM with deletion17p and t(4;14) respectively.
We hypothesized that addition of Ixazomib (oral proteasome inhibitor) at increased dose density to Pd (IxPd) in HR RRMM would improve convenience, thus adherence to treatment, and in parallel improve efficacy with no increased toxicity compared to addition of a parental proteasome inhibitor (PI).
Methods. Eligible patients had a RRMM, in L2, refractory to lenalidomide, but not to pomalidomide and ixazomib. HR was defined by presence of either del(17p) and/or t(4;14) at diagnosis or study entry. Patients received 17 induction cycles, 21-days long, consisting of ixazomib 3mg/day (d 1, 4, 8 and 11), pomalidomide 4mg/day (d1 to 14) and weekly dexamethasone, followed by a maintenance phase of 28-day cycles with ixazomib 4mg/day (d 1, 8 and 15) and pomalidomide 4mg/day (d 1 to 21), until progression. The primary endpoint was time to progression (TTP). The number of patients to be recruited was initially calculated based on an expected doubling of the median TTP obtained in IFM 2010-02 in either 2 HR RRMM population. No statistical comparison can be done across HR groups given that the study was powered to analyze the 2 groups as a whole.
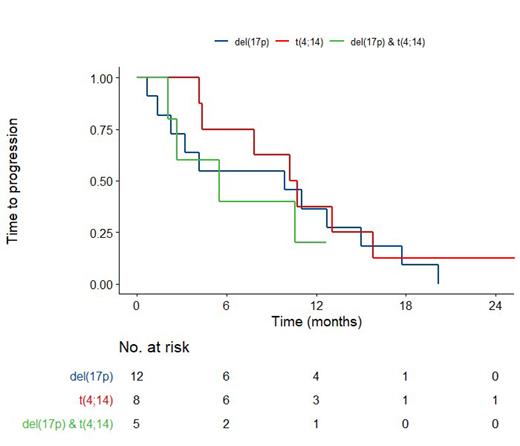
Results. Twenty-six patients were enrolled in the study. Median age at inclusion was 72 years (IQ. 67-78), median age at diagnosis was 70 years (IQ. 63-74). Twelve patients presented with del(17p), 9 with t(4;14), and 5 with del(17p) and t(4;14), all patients refractory to lenalidomide.
One patient received the study medication but died with no post baseline efficacy assessment. Analyses were done on the remaining 25 patients and safety analysis were performed on all the 26 patients having received at least one dose of the study medication. Twenty-two patients had progressed on study (of whom 12 had died at end of study), 2 patients died before progression, and one patient had no death and no progression. Study treatment was permanently interrupted in 9 patients before progression or death, including 8 during the induction phase.
With a median follow-up time of 27 months, the primary end-point median TTP for the efficacy analysis cohort (n=25) was 10.1 months (CI95%. 4.4;13). The median PFS and OS were 9.9 (CI95%. 4.2;12.7) months and 23.7 (CI95%. 12.7;inf) months, respectively. At end of induction, ORR and VGPR rate was (n=15) 60% and (n=7) 28%, CBR (n=18) 72%; at end of study the response rates were the same as end of induction.
The median TTP and OS for del(17p) was 9.9 (CI95%. 3.3;inf) and 23.7 (CI95%.14.3;inf ) months, respectively; and was 10.5 (CI95%. 7.9;inf) months and not reached (CI95%. 27.1;inf), in the t(4;14) cohort respectively. Median TTP and OS for combined del(17p) and t(4;14) was 5.5 (CI95%. 2.7;inf) and 11.1 (CI95%. 9.9;inf) months respectively.
The most common adverse events (10% occurrence) were neutropenia (n=15), thrombocytopenia (n=12), asthenia (n=10), anemia (n=8), rash (n=5), back pain (n=5), diarrhea (n=5), peripheral oedema (n=4), dizziness (n=3), general physical health deterioration (n=3), muscle spasms (n=3), pyrexia (n=3) and peripheral sensory neuropathy (n=3). There was no added safety concerns related to the combination of Ixazomib and Pomalidomide in the study protocol.
Conclusion. The study IFM 2014-01 /IxPd in HR L2 Len refractory RRMM met its primary end point objective since we observed a doubling of the median TTP with the addition of Ixazomib to pomalidomide and dexamethasone in this very hard to treat population characterized with a very poor outcome. This data confirms the importance of proteasome inhibitors in HR RRMM. This phase 2 study needs confirmation in a larger cohort.
Disclosures
Manier:Janssen: Honoraria; BMS: Honoraria; Amgen: Honoraria; Abbvie, Amgen, Celgene/BMS, GlaxoSmithKline, Janssen, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda: Membership on an entity's Board of Directors or advisory committees. Hulin:Amgen: Honoraria; Bristol Myers Squibb: Honoraria; Janssen: Honoraria; Sanofi: Honoraria; AbbVie: Honoraria; Pfizer: Honoraria. Karlin:AbbVie, Amgen, Celgene, Janssen, Sanofi, Takeda: Honoraria; Amgen, Celgene, GSK, Janssen, Takeda: Consultancy. Arnulf:Bristol Myers Squibb: Consultancy, Honoraria, Other: Meeting travel payments; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Meeting travel payments, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Meeting travel payments; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Meeting travel payments; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Meeting travel payments. Belhadj Merzoug:Amgen, Janssen, Pfizer, Sanofi, Takeda: Other: Travel Support; Amgen, BMS, Janssen, Sanofi: Honoraria; BMS: Research Funding. Decaux:Janssen, BMS, GSK, Sanofi, Takeda, Roche, Gilead: Honoraria. Sonntag:Janssen, Takeda, BMS and Sanofi: Membership on an entity's Board of Directors or advisory committees. Macro:Janssen, Takeda: Honoraria, Other: Travel/accommodation, Research Funding; GSK, Sanofi: Honoraria. Vincent:BMS, Takeda: Membership on an entity's Board of Directors or advisory committees, Other: Financing meeting participation; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Financing meeting participation; Pfizer: Other: Financing meeting participation. Touzeau:Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees. Perrot:Abbvie, Adaptive, Amgen, BMS, Janssen, Pfizer, Sanofi, Takeda: Honoraria. Moreau:GSK: Honoraria, Other: Advisory Board; janssen, celgene BMS, abbvie, sanofi, amgen, takeda, pfizer: Honoraria, Other: advisory boards. Leleu:Takeda: Honoraria; Merck: Honoraria; BMS/Celgene: Honoraria; Amgen: Honoraria; GSK: Honoraria; AbbVie: Honoraria; Janssen: Honoraria; Harpoon Therapeutics: Honoraria; Novartis: Honoraria; Sanofi: Honoraria.